The Measurements: Stable Isotope Mass Spectrometry
What is a Mass Spectrometer?
Meet Spock. Along with his nine other roommates, Spock is named after the character from the Star Trek series. But instead of residing in the USS Enterprise spaceship, Spock lives in a laboratory at INSTAAR in Boulder, Colorado. This laboratory contains air flasks waiting to be sampled, a wall lined with tanks of standards, large metal containers of liquid nitrogen, and computers showing graphs of carbon isotope ratios.
Like Spock from the original series, this Spock owes his creation to science. Spock is a highly precise isotope ratio mass spectrometer, which uses a magnetic field to separate and measure molecules of different masses (a measure of how much matter is in an object) by bending ions of different charges at different radii. Mass spectrometry is utilized in a multitude of fields ranging from medical characterization of proteins 4 to detecting pesticide amounts in foods and dietary supplements 5. Spock is specifically designed for a much narrower purpose: to examine carbon (and oxygen) isotopes in atmospheric carbon dioxide. The isotopic composition of each CO2 molecule determines its mass. By determining the relative concentrations of CO2 molecules of different masses, Spock can find the isotopic ratio in the sample.

Carbon dioxide comes in three different masses, depending on the isotopic makeup. Common garden variety CO2 (made up of one 12C and two 16O atoms) has a molecular mass of 44, whereas CO2 containing a 13C instead of 12C atom (and two 16O atoms) has mass 45. Additionally, CO2 containing an 18O instead of 16O oxygen isotope has mass 46. Of course there are a few CO2 molecules that contain a 13C and an 18O, or even two 18O atoms, but these are so rare that they are not usually measured. Spock measures these relative concentrations of the three main carbon dioxide isotopologues (molecules of the same material but with different isotopes in them) with molecular masses of 44, 45, or 46 within each air sample.
- Technical note: There is also a 17O isotope, which is much much rarer than 13C or 18O. Although 12C17O16O looks like 13C16O16O, a small correction is made for the contribution of this isotopologue.
| Mass | Isotopologues | Abundance |
|---|---|---|
| 44 | 12C16O2 | 98.40% |
| 45 |
13C16O2 12C17O16O |
1.19% 0.0748% |
| 46 |
12C18O16O 13C17O16O 12C17O2 |
0.41% 0.00084% 0.0000142% |
Spock was born in 1992. Despite his advanced age, Spock is still sharp, and he produces excellent high quality measurements, with a little bit of tweaking and cleaning once in a while. Back in 1992, Spock was easily able to keep up with all the air samples collected in the NOAA network. Lately though, NOAA scientists are collecting a lot more samples (over 7,000 a year), all of which are measured for stable isotopes, so Spock has a lot of trouble keeping up with the demand.
To learn more about Spock's home at the Institute of Arctic and Alpine Research's Stable Isotope Laboratory, visit INSTAAR's website at:
http://instaar.colorado.edu/sil/about/index.php.
The Process - What Spock Does
Before an air sample can be analyzed using isotope ratio mass spectrometry on Spock, carbon dioxide must be extracted and purified from the air flask so that carbon dioxide enters the mass spectrometer.
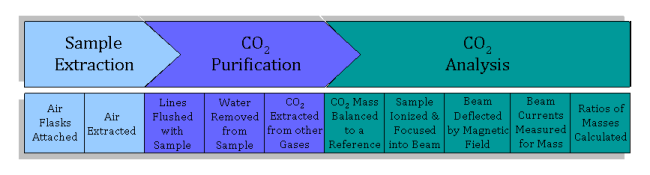
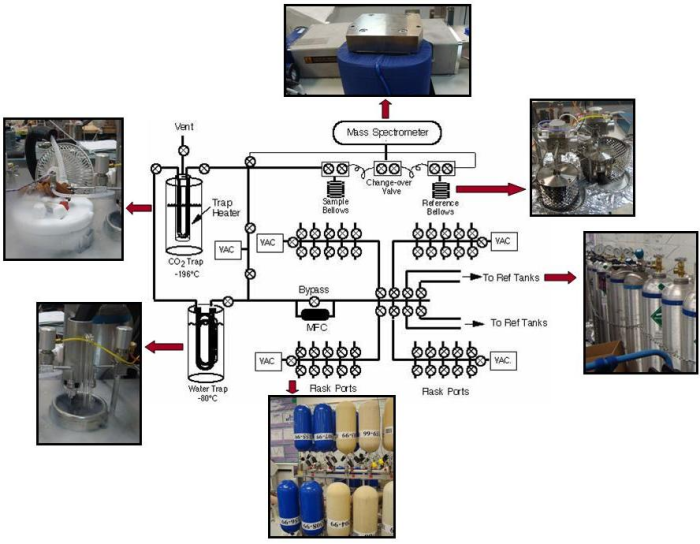
The first step is to extract a subsample from each air flask.
- Forty flasks at a time are attached to the flask ports. Two large tanks of atmospheric air which are used as standards are also attached. An automatic system allows one flask at a time (or one tank) to be opened.
- First, the lines through which the sample passes are evacuated before any sample air travels through the lines. During evacuation, any air inside the line (line is the term used to describe the sealed system of stainless steel tubing and valves that the sample air is put into, similar to a plumbing system, but with air instead of water inside) is removed using a vacuum pump (“under vacuum” means there is no air). This process is used to avoid contamination; gases mix very rapidly, so any air left over from the last sample or standard (or room air if the line was opened up for cleaning) must first be is removed from the line, ensuring that the air analyzed for its isotopic content actually comes from the original flask.
- The air is extracted into the line using a vacuum pump that slowly sucks or “pulls” a set amount of air (about half a liter) from the flask through the line into the carbon dioxide extraction system.
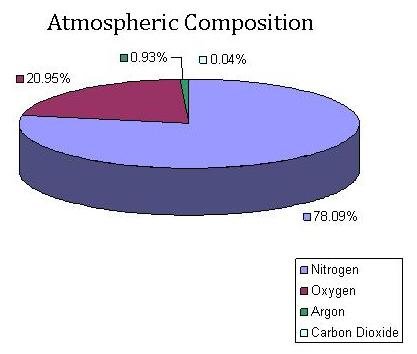
Although, at this point, the air sample has been successfully removed from its original flask, the sample is still not ready for isotope ratio mass spectrometry analysis. Along with the carbon dioxide in the air sample are other gases and water vapor. In fact, carbon dioxide makes up less than 1% of the gasses in our atmosphere.
The next step is to separate the carbon dioxide that enters the mass spectrometer.
- The sample is pulled through a glass water trap, where all water vapor is removed from the air sample. The water trap is simply two loops in the line that are chilled so that the water vapor freezes into ice on the walls and is therefore removed from the gas mixture. The loops are filled with small beads, which are used to increase the surface area of the loops, ensuring that all water vapor is removed. Completely removing water vapor from the sample is crucial in maintaining high precision, which is why there are two loops in the line through the water trap. Water vapor that is not trapped in the first loop is caught when the sample travels through the beads in the second loop.
-
The sample is then pulled through a CO2 trap, another loop in the line, which is cooled using liquid nitrogen (at an incredibly cold temperature of -196°C or -321°F). The sample becomes so cold that the gaseous CO2 freezes into dry ice whereas all the other gases stay in gas phase and pass through to the pump and out into the room–separating the carbon dioxide from the other gases. The sample is isolated from the other gases and then warmed, so that gaseous CO2 remains in the sample.
- Technical note – N2O is trapped too, and although it contains no carbon, it has the same mass as CO2. It's much less abundant than CO2, and its concentration is measured along with CO2 and other trace gases at one of NOAA's Carbon Cycle measurement laboratory, after which the scientists can correct the results for the measured amount of N2O.
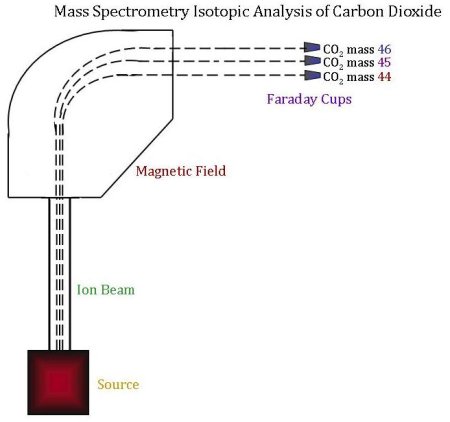
The sample is now ready to be analyzed for its isotopic content using a mass spectrometer. In order to determine relative concentrations of the different isotopes by mass spectrometry:
- After the sample is warmed, it is transferred into one of two bellows in the isotope ratio mass spectrometer. The bellows are simply a volume where the gas is stored. They have an accordion shape, and can expand or contract in order to control the pressure of the gas. The second bellows contains a reference gas, which also consists of CO2. The amount of CO2 from each sample varies, so the bellows are expanded and contracted to keep the pressure inside both the sample and reference bellows the same.
- The CO2 sample and standard alternatively flow into the ion source. The ion source is located in the analyzer, which is kept under vacuum so that no outside air can enter the source. In the source, the CO2 is bombarded with a beam of high-energy electrons. This causes the molecules to be ionized, after which they are accelerated and focused into an ion beam.
- The ion beam is then deflected by a magnetic field. When deflected, ions of different masses bend slightly differently since it is harder to bend a bigger mass. This creates three ion beams for carbon dioxide, corresponding to CO2 of mass 44, 45, and 46.
- The radius at which each mass bends is already known, and three Faraday cups are set in the right places to measure each ion beam. A Faraday cup is a metal cup located (once again) in a vacuum; it catches charged particles (ions), and these ions produce an electrical current inside the cup, which is proportional to the total number of ions entering the cup.
- Finally, the three beam currents (for mass 44, 45 and 46) are compared and ratios of the three currents are calculated and compared to the reference gas. A mathematical comparison of these ratios yields the final product–the δ13C value for a given sample.
Precision and Quality
All stable isotope data are checked by the laboratory technicians to ensure that it is of the highest quality possible. Not only are the paired flasks checked for agreement among each other, but measurements of flasks are compared among labs. Additionally, the INSTAAR laboratory personnel run standards as samples to make sure that the mass spectrometer yields the expected ratios.

 Previous
Previous
