The Technical Details: Radioactive Decay
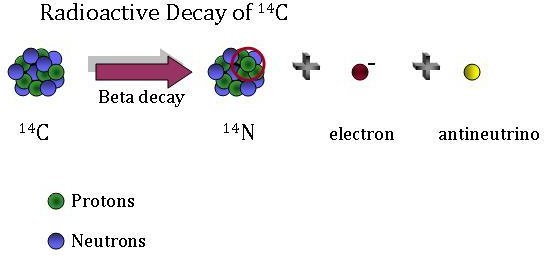
Beta Decay
Because 14C is radioactive, it decays over time–in other words, older artifacts have less 14C than younger ones. 14C decays by a process called beta decay. During this process, an atom of 14C decays into an atom of 14N, during which one of the neutrons in the carbon atom becomes a proton. This increases the number of protons in the atom by one, creating a nitrogen atom rather than a carbon atom. An electron and an elementary particle, called an antineutrino, are also generated during this process.
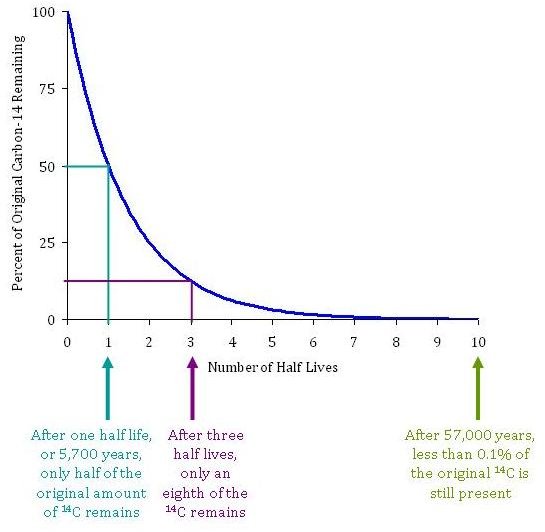
The Concept of a Half Life
The time it takes for 14C to radioactively decay is described by its half-life. 14C has a half-life of 5,730 years. In other words, after 5,730 years, only half of the original amount of 14C remains in a sample of organic material. After an additional 5,730 years–or 11,460 years total–only a quarter of the 14C remains. The amount of 14C remaining is used to determine the age of organic materials. After 10 half-lives, or 57,300 years, the amount of 14C remaining (<0.1%) becomes very difficult to detect. Thus fossil fuels, which are much much older than 50,000 years, have no 14C remaining.
For more information on the history of radiocarbon dating, its usage in climate change studies, and a brief description of other fields that rely on radiocarbon dating, visit The American Institute of Physics link:
http://www.aip.org/history/climate/Radioc.html
http://www.c14dating.com/applic.html
If 14C is Always Decaying, Why is it Still in Our Atmosphere?
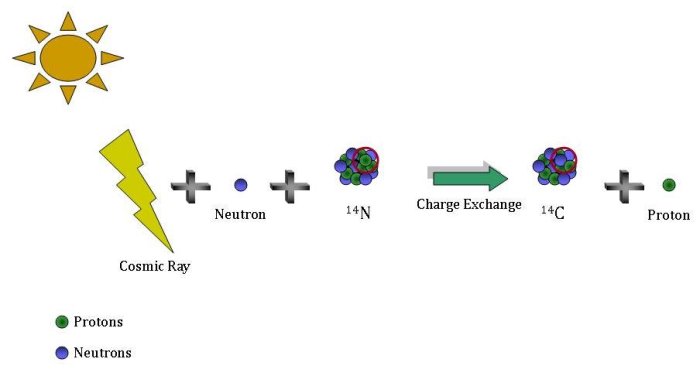
How is it that there is still 14C left in the atmosphere (or anywhere else on Earth) when it is constantly disappearing? Where does new 14C come from?
Cosmic rays are high energy particles that originate in outer space. When they collide with matter in the atmosphere they can shatter a nucleus into smaller pieces (a process called spallation), including neutrons. The latter slow down, again by colliding with matter in the atmosphere. Once they have slowed down enough a neutron can be absorbed by a nitrogen-14 (14N) nucleus while kicking out a proton, resulting in a 14C nucleus.