Stimulated Raman Scattering
A Simple Way to Change the Color of a Laser
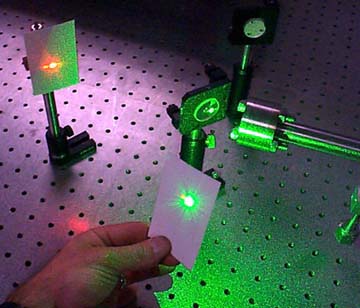 Lasers are really useful tools for making atmospheric
measurements since they have a unique color and are so bright. They can
be pointed into the sky and used to determine how windy it is or how much
pollution is in the air. Having different color lasers allows us to study
the atmosphere easier that having only a single colored laser. It's like
having a whole set of different color pencils for making a drawing. You
can do it with one color but having many colors paints a better picture.
Lasers are really useful tools for making atmospheric
measurements since they have a unique color and are so bright. They can
be pointed into the sky and used to determine how windy it is or how much
pollution is in the air. Having different color lasers allows us to study
the atmosphere easier that having only a single colored laser. It's like
having a whole set of different color pencils for making a drawing. You
can do it with one color but having many colors paints a better picture.
Color is Wavelength
In the world of physics we can't use the word red to describe a color very well because there are so many reds; in fact there are infinite variations of red. A quantity called wavelength is used which is the physical length of one cycle of a light wave. The length of a cycle of light is so short that the wavelength is typical expressed in nanometers (1 billionth of a meter) or nm for short. The red of a helium neon laser has a wavelength of 632.8 nm. The red of a diode laser pointer has a wavelength of 675 nm. We generally work with lasers in the ultraviolet which have a wavelength of less than 300 nm. Your eyes can see light in the wavelength range of 400-700 nm.
One of the ways to get different colors is to use a techniques called "Stimulated Raman Scattering." This techniques is named after a scientist named Dr. Raman who discovered that light can change color when it interacts with molecules. Molecules in everything vibrate whether they are in a pencil, the air you breath, or in your body; they are always moving. The warmer something is, the more the molecules vibrate. Something really hot has molecules that vibrate a lot and something very cold has molecules that vibrate less. If light hits some of these vibrating molecules, sometimes a molecule can steal some of the light's energy. When this happens, the light changes color. The opposite can also happen; the molecule can give the light some energy. This also changes the light's color. Basically, any change in energy of light, changes its color. When the molecule steals the light's energy, it vibrates more and when it gives the light energy, it vibrates less. All these events must obey the law of conservation of energy.
How we are using stimulated Raman scattering at ESRL
We are using Stimulated Raman Scattering techniques to make other colors to help monitor ozone pollution. To do this, we take a long metal tube with window on each end (also called a cell) and fill it up with a gas such as hydrogen or deuterium. The gas is put in at a pressure of several hundred PSI (pounds per square inch). The pressure of the gas plays a very important role in the results. A laser is focused into the tube and the light comes out a different color (There's always some of the original color light left over). The picture below shows how this experiment is set up.

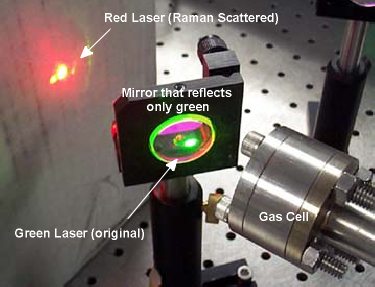 To demonstrate how stimulated Raman scattering works,
a green laser at 532 nm is focused into a cell of deuterium. The molecules
of deuterium vibrate in such a way that the light changes into red (632
nm). Since red light has less energy than green light, the deuterium molecules
take some of the green light's energy away in the process. The picture to
the right shows what this looks like in the lab. A special mirror that only
reflects green light is used to separate the two colors.
To demonstrate how stimulated Raman scattering works,
a green laser at 532 nm is focused into a cell of deuterium. The molecules
of deuterium vibrate in such a way that the light changes into red (632
nm). Since red light has less energy than green light, the deuterium molecules
take some of the green light's energy away in the process. The picture to
the right shows what this looks like in the lab. A special mirror that only
reflects green light is used to separate the two colors.
Hydrogen makes the laser light change to a particular color and deuterium makes it change to a different color. By using different types of gases, we can get a wide variety of colors. In particular we are interested in getting two different colors at the same time so we mix both hydrogen and deuterium together in one cell. This lets us use one cell to get the colors that are important for measuring ozone pollution.