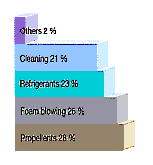DEFINING THE THREAT TO THE OZONE LAYER
Until the early 1970s nobody dreamed that human activity could threaten to deplete the ozone layer. Then scientists identified two potential problems: exhausts from supersonic planes (SST) flying in the lower stratosphere, and chemicals used in refrigerators and as propellants in spray cans. In 1971, H. S. Johnston, at the University of California (Berkeley), pointed out the potential danger of a large fleet of SSTs emitting considerable amounts of nitric oxide into the lower stratosphere, possibly accelerating natural ozone destruction. Only three years later, F. S. Rowland and M. Molina showed that a widely used class of very inert chemicals known as chloro- fluorocarbons were transported to the stratosphere by convective air movements. There, they could absorb high-energy photons from sunlight and release free chlorine; Once released, the chlorine could destroy stratospheric ozone through a series of catalytic reactions.
We now know that bromine from the halons used in some fire extinguishers can also be released in the stratosphere, with even greater ozone -destructive effect. Some CFCs and halons can survive in the atmosphere more than a century. Moved by air currents, the halocarbons released over the past sixty years are a threat to the ozone layer for decades to come. They are carrying thousands of tons of chlorine and bromine atoms into the stratosphere. This is many times greater than the chlorine reaching the stratosphere naturally from the ocean in the form of methyl chloride and bromide.
|
|
|
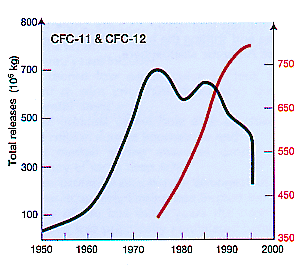
HALOCARBONS Halocarbon is a generic term covering a number of human-produced gases all containing carbon and halogen (fluorine, chlorine or bromine) atoms. Halocarbons include chlorofluocarbons (CFCs) and halons. The first was synthesized in 1928. Since then, they have come to be widely used for a variety of purposes such as propellants in aerosol cans. in the manufacture of soft and hard foams, in refrigeration and air conditioning, and as cleaning solvents (Figure 4). Consequently, they were increasing rapidly in the atmosphere (Figure 3). Halocarbons in the troposphere are inert, non-toxic. non-flammable. odourless and colourless. However, when they reach the stratosphere, particularly at and above the layer of maximum ozone (19-23 km), high-energy ultraviolet photons from the Sun free chlorine or bromine atoms. These atoms catalytically detach one oxygen atom from an ozone molecule, thus convert ozone to molecular oxygen (Figure 6 and Figure 7).
|
In 1975, WMO convened a group of experts to prepare an authoritative statement entitled "Modification of the ozone layer due to human activities and some possible geophysical consequences". The statement focused on the effects of both supersonic transport and CFCs. It signalled the first international warning of the danger of substantial ozone decrease and recommended international action to provide better understanding of the issue.
The following year WMO launched the Global Ozone Research and Monitoring Project to provide advice to Members, the United Nations and other international organizations concerning:
- The extent to which anthropogenic pollutants might be responsible for
reducing the quantity of ozone in the stratosphere;
- The possible impact of changes in stratospheric ozone on climate trends
and on solar UV radiation at the Earth's surface;
- Identification of needs for strengthening the long-term monitoring of ozone.
Research by hundreds of scientists sponsored by government organizations from around the world has enhanced knowledge of the threat to the ozone layer. Along with increased collaboration with UNEP, this has substantially helped successful implementation of WMO's Ozone Project. It has produced 38 substantial scientific reports, including six major assessments of the ozone layer, which provided the basis for the preparation of the international ozone treaties and their amendments.
In 1984, at the Ozone Commission Symposium in Halkidiki, S. Chubachi (Japan Meteorological Agency) reported observing extremely low ozone values (about 200 m atm cm) at Syowa during many days of the 1982 Antarctic spring. The full significance of that data was recognized only after the publication in 1985 of data from a British Antarctic Survey station at Halley showing the dramatic decline of ozone an actual "hole" in the ozone layer forming each spring since the early 1980s.
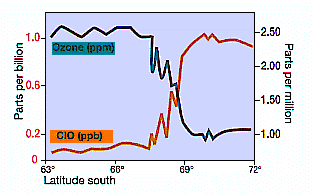
The majority of scientists thought this was the first sign that halocarbons (see box) were eroding the protective ozone layer. There was uncertainty until measurements procured by special expeditions in 1986 and 1987 documented the presence of reactive chlorine species (eg. CIO see Figure 5). These and subsequent measurements have demonstrated that human-produced CFCs are depleting the ozone and shifting the fragile chemical balance of the Antarctic Stratosphere.
|
Figure 5 -- Measurements of ClO concentration and ozone from aircraft (NASA, 1987). Note the rapid incease of ClO as the aircraft enters the polar votex and the Antactic ozone 'hole' (about 67°S) and the inverse correlation of ClO with ozone decline in mid-September when the chemically- unbalanced area in the vortex was sunlit |