Scientific Assessment of Ozone Depletion: 1994
Common Questions About Ozone

3. Does Most of the Chlorine in the Stratosphere Come from Human or Natural Sources?
Most of the chlorine in the stratosphere is there as a result of human activities.
Many compounds containing chlorine are released at the ground, but those that dissolve in water cannot reach stratospheric altitudes. Large quantities of chlorine are released from evaporated ocean spray as sea salt (sodium chloride) aerosol. However, because sea salt dissolves in water, this chlorine quickly is taken up in clouds or in ice, snow, or rain droplets and does not reach the stratosphere. Another ground-level source of chlorine is its use in swimming pools and as household bleach. When released, this chlorine is rapidly converted to forms that dissolve in water and therefore are removed from the lower atmosphere, never reaching the stratosphere in significant amounts. Volcanoes can emit large quantities of hydrogen chloride, but this gas is rapidly converted to hydrochloric acid in rain water, ice, and snow and does not reach the stratosphere. Even in explosive volcanic plumes that rise high in the atmosphere, nearly all of the hydrogen chloride is scrubbed out in precipitation before reaching stratospheric altitudes.
In contrast, human-made halocarbons - such as CFCs, carbon tetrachloride (CCl4) and methyl chloroform (Ch3CCl3) - are not soluble in water, do not react with snow or other natural surfaces, and are not broken down chemically in the lower atmosphere. While the exhaust from the Space Shuttle and from some rockets does inject some chlorine directly into the stratosphere, this input is very small (less than one percent of the annual input from halocarbons in the present stratosphere, assuming nine Space Shuttle and six Titan IV rocket launches per year).
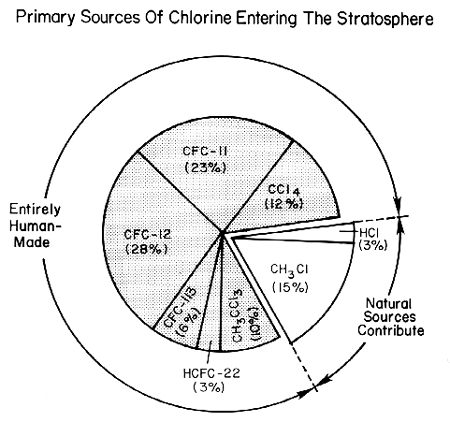
Several pieces of evidence combine to establish human-made halocarbons as the primary source of stratospheric chlorine. First, measurements (see the figure below) have shown that the chlorinated species that rise to the stratosphere are primarily manufactured compounds (mainly CFCs, carbon tetrachloride, methyl chloroform, and the HCFC substitutes for CFCs), together with small amounts of hydrochloric acid (HCl) and methyl chloride (Ch3Cl) which are partly natural in origin. The natural contribution now is much smaller than that from human activities, as shown in the figure below. Second, in 1985 and 1992 researchers measured nearly all known gases containing chlorine in the stratosphere. They found that human emissions of halocarbons plus the much smaller contribution from natural sources could account for all of the stratospheric chlorine compounds. Third, the increase in total stratospheric chlorine measured between 1985 and 1992 corresponds with the known increases in concentrations of human-made halocarbons during that time.